Post a comment on the text below
3.6 Contamination from metals - mining and use
Metals have been used for centuries in many different applications. As well as leading to high concentrations in naturally metalliferous areas, their extraction and processing have led to polluted districts - even long after mines have closed down (Box 3.3). Widespread use of metals in industry, and subsequent discharge to water also continues to cause pollution, as metals are transported within the water column and its sediments.
Box 3.3: Ancient Mining in the Harz Mountains in Germany
Metals e.g. lead and cadmium exceed the EQS in the Harz Mountains foothills of Weser River in northern Germany. For centuries, it was one of important ore mining regions of Germany. The mining activity mainly closed down in the 1930s and in 1992 the last mine closed. Around the mines are a large number of tips and chemical and metallurgic industry. The rivers most contaminated are some tributaries of the Leine River, which has a catchment area of about 6.500 km². Metal contamination down the river is visible until the estuary of the Weser in the North Sea.
The river and floodplain sediments have been contaminated with the waste and mine water over centuries. In the floodplains high lead and cadmium concentrations affect the agriculture at pasture and arable land along the river floodplains. Livestock farming and agriculture is possible in limited form only. [Owing to the large area affected,] decontamination would be very difficult and lowering concentrations of the metals in the rivers represents a long term effort (FGG Weser, 2016). Similar contamination and effects on waters are seen all over Europe in old mining regions.
Map 3.2, Metal pollution from mining areas in the Harz catchment.
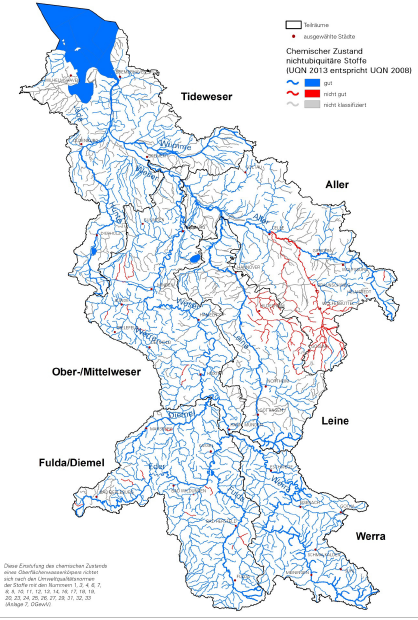
Source: http://www.fgg-weser.de/component/jdownloads/send/8-eg-wrrl/331-bwp2015-weser-final-textteil-160318
Table 3.1c: List of pollutants most frequently exceeding EQS in EU25
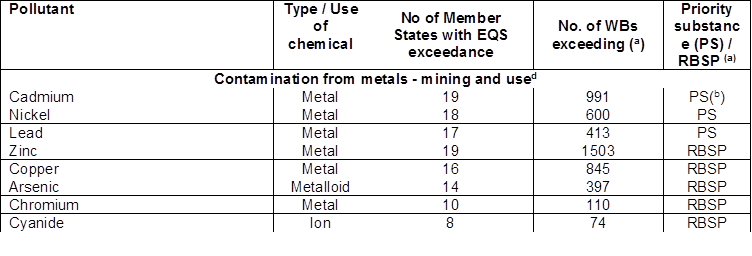
(a) under the WFD EU-wide standards apply for priority substances (PS), while national or river basin standards apply for River Basin Specific Pollutants (RBSP).
(b) defined as priority hazardous substances, for which all discharges, emissions and losses must be ceased.
Sources and uses
Metals are natural substances and have been mined for centuries and used in many different ways, from producing tools, vehicles and buildings to sophisticated applications in industrial processes, as well as numerous domestic applications. Some historic uses shown to be particularly harmful, and so restricted, include the use of lead in water pipes and as a petrol additive. (Mercury is discussed in section 3.4.)
Metals reach the aquatic environment in many ways, reflecting their multiple uses. Rainfall may leach them from mines, industrial or waste sites, they may be discharged in effluents to sewers or directly into rivers, lakes, etc. Being natural elements, metals do not degrade, although they can be converted to other forms which may be more or less harmful. Many dissolved metals can bind to suspended material and sediment and be transported downstream, or recycled within a water body.
Toxicity and EQS
Since metals occur naturally in the environment and some metals are essential elements for living beings, it is not always easy to assess when concentrations start having negative or even toxic effects. These can vary for individual species and the environment conditions.
The solubility and bioavailability of metals are influenced by pH, organic compounds naturally present in water (such as humic substances) and calcium. Ecotoxicological effects are exacerbated in soft water (i.e. low lime content) and low pH. Increasing knowledge about the detrimental impacts of metals have led to extensive monitoring and research into the ecotoxicological effects. Modelling of metals under such differing conditions has been undertaken to assess their bioavailability, allowing assessment of measured concentrations with the bioavailable concentration. This can be used to target measures where the metals present most risk to aquatic organisms. The 2013 Priority Substances Directive included bioavailable EQS for nickel and lead.
The EQS for cadmium and lead are set to protect invertebrates, while that for nickel is set to protect algae and molluscs.
WFD status
Among the 15 priority substances most frequently causing failure to achieve good chemical status are the metals mercury (discussed in section 3.4), cadmium, lead and nickel. This may reflect a relatively high level of reporting for metals, with approximately 2/3 Member States reporting failures to achieve chemical status for these substances. An additional five metals – zinc, copper, arsenic, chromium and cobalt - are among the most frequently-reported RBSPs causing failure of ecological status. There were more failures in surface water bodies for zinc and copper than for many of the priority substances.
Despite widespread use, failures to achieve good chemical status for cadmium, lead and nickel range from 413-991 (table 2.1) in surface water bodies. Member States are making progress with these metals - 969 water bodies improved from poor to good chemical status from the first RBMPs, though 2288 water bodies were still failing (EEA, 2018a).
Emissions
Figures 3.9-3.11 give an overview of the loads reported under different mechanisms.
Figure 3.9 : Cadmium
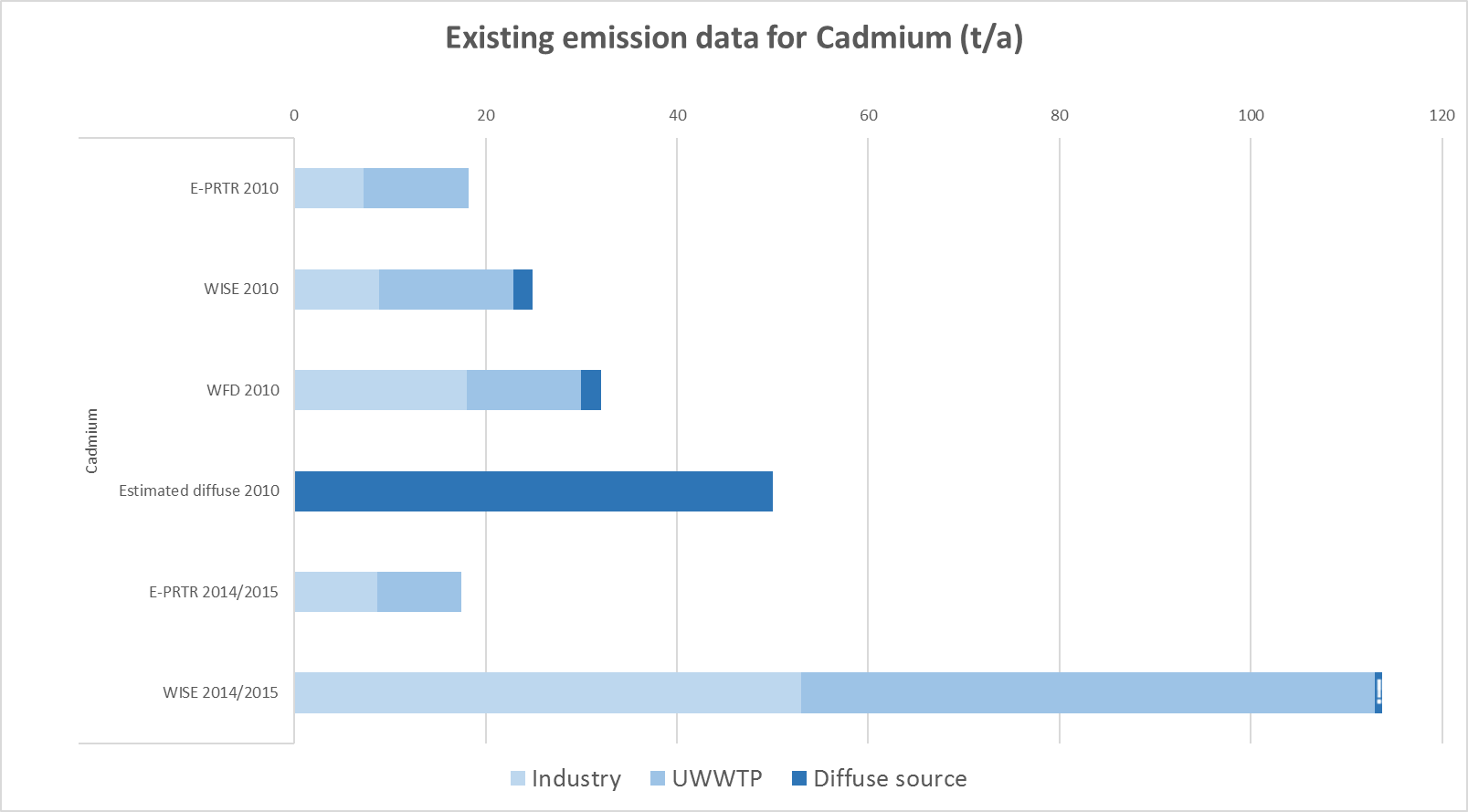
Figure 3.10 : Nickel

Figure 3.11 : Lead

Loads given in these figures cannot be summed, as there may be double counting.
For other metals, there are limited, comparable, emissions data as the WFD inventory only includes priority substances. Further information on E-PRTR reported emissions of zinc and copper are available at https://www.eea.europa.eu/themes/industry/industrial-pollution/ and in Roovaart et al. (2017).
There is a high level of reporting of metals for emissions from industry and UWWTPs. UWWTPs are the largest known source for cadmium and nickel, while for lead it is industry. However, Roovaart et al. (2013) suggested that there was significant under-reporting for emissions from UWWTPs for all three metals. Reflecting the widespread use of metals, countries reported a range of diffuse sources from agriculture, atmospheric deposition, unconnected households, storm water overflows, transport and run-off.
However, despite high levels of reporting of metals emissions, the overall trend is not clear, with high variability from year to year.
Between 2007 and 2014, arsenic and copper emissions reported under the E-PRTR for industry excluding UWWTPs showed no clear trend, while there was a decrease in zinc emissions (Roovaart et al, 2017). For UWWTPs reporting under E-PRTR, there was a slight increase in copper and zinc emissions, with a large increase in reported arsenic emissions from one country.
Summary/Outlook
Both research into the behaviour of cadmium, lead and nickel in the aquatic environment, and regulation around that, has been undertaken for decades. While there are still a significant number of surface water bodies failing to achieve good chemical status for metals, there are promising signs that further improvements can be made.
Forthcoming challenges may include the behaviour of metals as “co-contaminants”, where their presence at low levels may exacerbate the toxicity of other chemicals present in the same water body (chapter 2).
You cannot post comments to this consultation because you are not authenticated. Please log in.



Previous comments
DE-UBA II 2.2:
Box 3.3: Map title:
Actually, this map shows the whole Weser catchment not only the Harz as indicated in the title of the map.
DE-UBA II2.2:
'UWWTPs are the largest known source for cadmium and nickel, while for lead it is industry.'
In Germany diffuse sources (pathway) are much more important.
DE-UBA II 2.2:
'However, despite high levels of reporting of metals emissions, the overall trend is not clear, with high variability from year to year.'
Could the variability be due to hydrology?
DE-UBA II 2.2:
'
Between 2007 and 2014, arsenic and copper emissions reported under the E-PRTR for industry excluding UWWTPs showed no clear trend, while there was a decrease in zinc emissions (Roovaart et al, 2017). For UWWTPs reporting under E-PRTR, there was a slight increase in copper and zinc emissions, with a large increase in reported arsenic emissions from one country.'
Because of pollutant theresholds we find this statement difficult. There mignt be facilities where emissions vary around the threshold value (that means in one year reporting is necessary while in the next year it is not). Therefore, it is not known for sure if emissions de- or increased. We only know if reported emissions de- or increased.
Eurometaux
We suggest replacing the header “Contamination from metals – mining and use” with “Metals and cyanide”. The current header is misleading: it gives the impression that mining continues to be an important source of metal emissions, whereas metals mining in Europe is quite limited nowadays and most of the metal emissions from mines are, in fact, legacy contaminations from the past (as illustrated by the Box 3.3). While historical mining sites may still be significant sources of metals to local waters, several metals may have other dominant sources than “mining and use”: for example, natural occurrence (local metalliferous geology), smelting and refining, transport, fossil fuels, agriculture, … As correctly indicated by Figure 3.1, emission sources are a very complex picture, and this should not be over-simplified in the section headers.
Eurometaux
"their extraction and processing have led to polluted districts"
Have led: suggested “have historically led”
Eurometaux
Map 3.2, Metal pollution from mining areas in the Harz catchment.
Even prior to mining, naturally elevated concentrations of metals would have been associated with the deposits subsequently discovered in the mountains - and subject to natural weathering and erosion processes over centuries before mining began. It is important to clarify with the German authorities and geological survey to what extent they have been able to distinguish between natural erosion and deposition of metal-bearing minerals from the natural rock and erosion and deposition from mine workings and waste deposits.
Eurometaux
Table 3.1c
In fact, the importance for a bioavailability correction was highlighted in the JRC 2016 report (Monitoring based exercise: Second review of the Priority Substances list under the Water Framework Directive), using Zn as a case study. Results showed that incorporating bioavailability significantly lowered the STE risk score for Zn.
The aforementioned JRC 2016 report showed that Cu STE score was derived from 104,254 inland water dissolved samples, and Zn from 87,895. We suggest the number of samples for each substance, as well as the total no. of waterbodies, are listed in Table 3.1c.
Cyanide
Cyanide is not a metal and mining is not the source.
Eurometaux
Sources and uses
"Metals reach the aquatic environment in many ways, reflecting their multiple uses."
"metals do not degrade"
It should also be acknowledged that metals reach the aquatic environment naturally - even without their being used.
Metals occur in combination with other elements in naturally occurring minerals - which do weather and degrade; the sentence “metals do not degrade” is therefore misleading.
Eurometaux
"The EQS for cadmium and lead are set to protect invertebrates, while that for nickel is set to protect algae and molluscs."
The report states that the Cd and Pb EQS is set to protect invertebrates, and that the Ni EQSs is set to protect molluscs and algae. This is not the case at all. All EQSs are set to protect aquatic ecosystems, not specific members of those ecosystems. In particular, the Ni EQS is based on a database comprising 31 species that includes algae, vascular plants, invertebrates, fish, and amphibians. The statement may be trying to say that molluscs and algae are the most sensitive organisms to Ni, but this is not the case, either. The top ten most sensitive species include molluscs, crustaceans, and vascular plants.
Despite widespread use, failures to achieve good chemical status for cadmium, lead and nickel range from 413-991 (table 2.1) in surface water bodies. Member States are making progress with these metals - 969 water bodies improved from poor to good chemical status from the first RBMPs, though 2288 water bodies were still failing (EEA, 2018a).
Comment Belgium (Wallonia) : same remarks as above: for lead and nickel, in the first reporting of RBMPs, EQS were set for soluble concentrations whereas now EQS are set for the bioavailable part of these concentrations calculated through simplified BLM (Biotic Ligand Models). This difference between the first and the second reporting of RBMPs could explain a part of the observed “improvement”.